Carnot Engine and Carnot Cycle
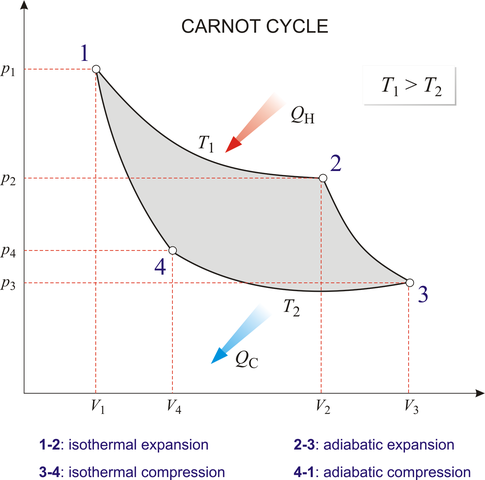
Carnot engine is a reversible engine of maximum efficiency. It operates between a hot reservoir at temperature T1 and a cold reservoir at temperature T2.
- Isothermal expansion: $(p_1,V_1,T_1)\to (p_2, V_2, T_1)$. The heat absorbed in this process is $Q_1$ and work done by the gas is \begin{align} W_{1\to2}=nRT_1 \ln(V_2/V_1) \end{align}
- Adiabatic expansion: $(p_2,V_2,T_1)\to(p_3,V_3,T_2)$. The work done by the gas is \begin{align} W_{2\to3}=nR(p_2V_2-p_3V_3)/(\gamma-1) \end{align}
- Isothermal contraction: $(p_3,V_3,T_2)\to (p_4, V_4, T_2)$. The heat released in this process is $Q_2$ and work done by the gas is \begin{align} W_{3\to4}=nRT_2 \ln(V_4/V_3) \end{align}
- Adiabatic contraction: $(p_4,V_4,T_2)\to(p_1,V_1,T_1)$. The work done by the gas is \begin{align} W_{4\to1}=nR(p_4V_4-p_1V_1)/(\gamma-1) \end{align}
I encorage you to draw Carnot cycle on T-S (temperature-entropy) diagram.
Solved Problems from IIT JEE
Problem from IIT JEE 2018
One mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where $V$ is the volume and $T$ is the temperature). Which of the statements below is (are) true?- Proecess I is an isochoric process.
- In process II, gas absorbs heat.
- In process IV, gas releases heat.
- Process I and III are not isobaric.
Solution: The volume is constant in an isochoric process. It is parallel to $T$ axis on a $T\text{-}V$ diagram.
The process II is isothermal expansion as temperature is constant and volume is increased. The change in internal energy of a gas in an isothermal process is zero i.e., $\Delta U=0$. The work done by the gas in an expansion process is positive i.e., $\Delta W=\int p \mathrm{d}V > 0$. By first law of thermodynamics, $\Delta Q=\Delta U+\Delta W=\Delta W > 0$, i.e., heat is absorbed by the gas.
The process IV is isothermal compression as temperature is constant and volume is decreased. Thus, $\Delta U=0$, $\Delta W<0$ and $\Delta Q=\Delta W<0$ i.e., heat is released by the gas.
The pressure is constant in an isobaric process. It is a straight line, $T=(p/nR)V$, on a $T\text{-}V$ diagram. Can you relate the given cycle with Carnot cycle?
Questions on Carnot Engine
Question 1: The free expansion of a gas is an irreversible process.
Question 2: Which of the following statement is FALSE about a Carnot engine?
Exercise
- A heat engine operates in a Carnot cycle. Mark True/False
- Throughout the operation the gas does positive work
- Throughout the operation the gas absorbs heat
- Throughout the operation the internal energy of the gas remains the same
- In integral number of cycles the gas does positive work
- In integral number of cycles the gas absorbs heat
- In integral number of cycles, the internal energy of the system remains the same.
