Thought provoking problems on heat and thermodynamics
These problems are from pre-test conducted in teacher's workshops by Prof HC Verma and his team.
Test 1
Source: RESPECT KV MRP Workshop 15th-24th May 2017
- Which of these is a function of the state of a gas?
- Heat $Q$
- Work $W$
- Internal energy $U$
- $U+PV$
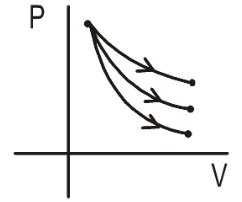
- The figure shows adiabatic processes for samples of equal moles of three gases. Write the name of one possible gas for each of the three processes. Process-1 gas:__________. Process-2 gas:_________. Process-3 gas:_______
- Consider isothermal expansion of an ideal gas.
- If the gas does a work W, how much heat Q is given to the gas?
- Is the heat Q completely converted into work in this process?
- Is this a violation of the 2nd law of thermodynamics?
- Consider the following gas systems all at room temperature. In which case $|pV-nRT|$ will be smallest and in which case it is largest?
- gas in an LPG cylinder
- hydrogen gas filled in a balloon which tries to go up in atmosphere
- air in your water bottle
- air in the tubes of a car
- A suit of a hotel has two identical rooms connected by a door. The living room is air conditioned and is set at 24 deg C while the outer room has no air conditioning. Suppose the door remains open for quite some time. Which of the rooms will have more air if it is
- a hot summer day (outside temperature 45 deg C),
- a chill winter night (outside temperature 5 deg C)?
- Consider an air molecule in a closed plastic box. What is the order of distance moved by this molecule in an hour?
- 1 km
- 10 km
- 100 km
- 1000 km
- 10000 km or more
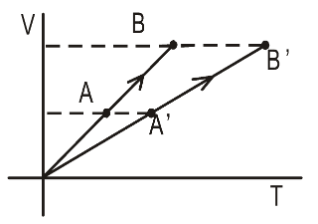
- Two processes $AB$ and $A^\prime B^\prime$ on a sample of ideal gas are shown on volume vs temperature diagram. In which of the two processes, the work done by the gas is larger? Give a one-sentence justification.
- A gas is suddenly compressed. During the process what kind of relation between the pressure and the volume should be used?
- Most of the solids expand on increasing temperature. This is because
- Some of the atoms leave the lattice site at higher temperature
- The vibration amplitudes of the atoms increase at higher temperature
- The inter-atomic potential energy is not symmetric about the minimum energy
- The electrons go to larger orbits.